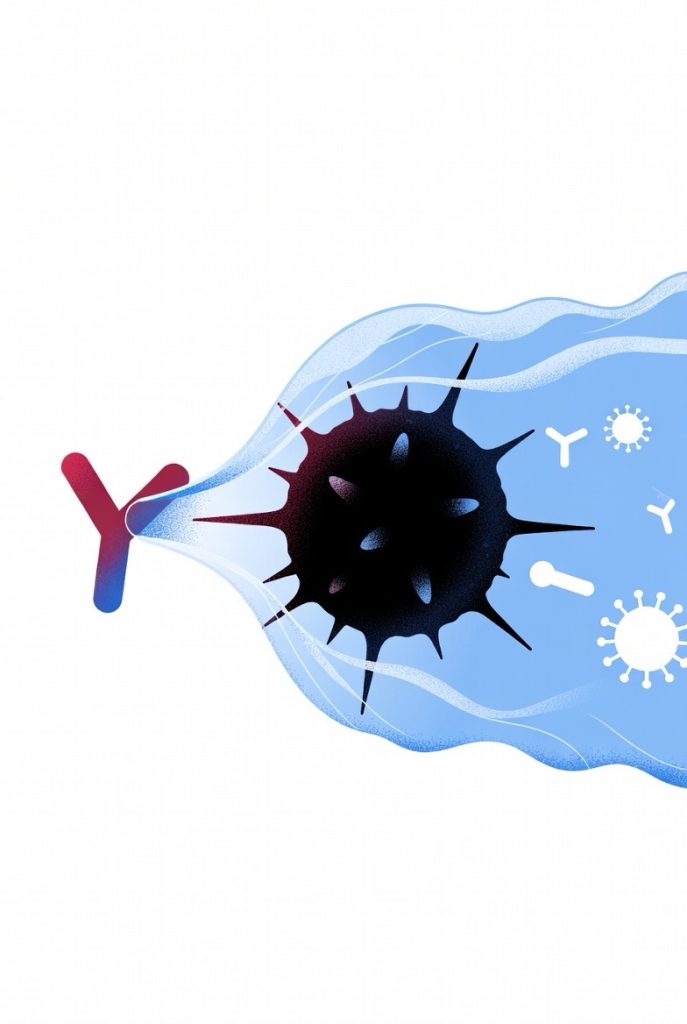
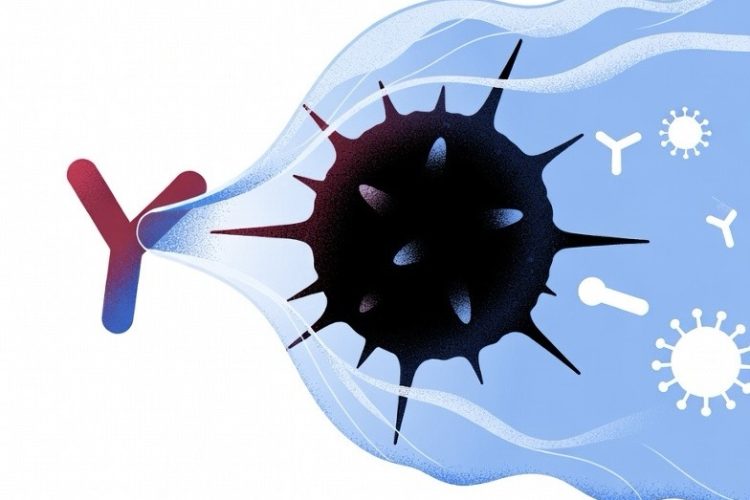
In 2026, MIT and Stanford researchers introduced AbLecs—antibody-lectin chimeras that remove the sugar coating (glycans, including sialic acids) cancer cells use to hide from the immune system. By blocking these glycans from binding to Siglec receptors, AbLecs disable glyco-immune checkpoints, exposing tumor-associated antigens and enabling immune cells like macrophages to recognize and destroy tumors. Preclinical studies showed AbLecs, paired with antibodies such as trastuzumab (HER2), rituximab (CD20), and cetuximab (EGFR), significantly boosted immune attack and reduced lung metastases in mouse models, even in low-antigen tumors. This modular approach offers a universal way to enhance immunotherapy across many cancer types, potentially overcoming resistance to PD-1 inhibitors. Valora Therapeutics is now advancing AbLecs toward clinical trials, promising broader, more effective cancer treatments.
Long Version
Revolutionizing Cancer Immunotherapy: AbLecs Unmask Tumors by Targeting Glyco-Immune Checkpoints
In the ongoing battle against cancer, a groundbreaking advancement has emerged from collaborative efforts between MIT and Stanford researchers. This 2026 breakthrough introduces AbLecs—antibody-lectin chimeras designed to strip away the sugar coating, or glycans, that tumors use to evade the immune system. By disrupting this camouflage, AbLecs enable immune cells to recognize and destroy cancer cells that were previously invisible, offering a universal strategy to enhance immunotherapy across multiple cancer types.
Understanding Cancer’s Immune Evasion Mechanisms
Cancer remains one of the leading causes of death worldwide, with tumors often outsmarting the body’s natural defenses through sophisticated mechanisms of immune evasion. Traditional treatments like chemotherapy and radiation can damage healthy tissues, while checkpoint blockade therapies, such as those targeting PD-1, have revolutionized care but only benefit a subset of patients due to resistance and immunosuppression. The immune system, comprising cells like macrophages and natural killer cells, is primed to attack invaders, but cancer cells exploit glyco-immune checkpoints to suppress this immune response.
At the heart of this evasion are glycans, complex sugar molecules on the surface of cancer cells. These include sialic acids, which bind to inhibitory receptors called Siglecs on immune cells, effectively putting the brakes on the immune attack. Tumor-associated antigens, often masked by these glycans, remain hidden, allowing cancers to proliferate and form metastases. Researchers have long sought ways to counteract this, and AbLecs represent a modular solution that combines the precision of antibodies with the glycan-binding power of lectins.
The Design and Functionality of AbLecs
Developed by Jessica Stark at MIT and Carolyn Bertozzi at Stanford, AbLecs are bispecific molecules that fuse a tumor-targeting antibody domain with a lectin “decoy receptor” domain. This design allows AbLecs to home in on specific cancer markers while directly binding and blocking sialic acids, preventing their interaction with Siglecs and lifting the immunosuppressive barrier. Unlike standalone lectins, which struggle to accumulate at tumor sites, the antibody component ensures targeted delivery, enhancing efficacy against immune evasion.
AbLecs function by selectively removing or blocking the glycan camouflage on cancer cells, thereby exposing tumor-associated antigens to the immune system. This process boosts the immune response, promoting antibody-dependent cellular phagocytosis and cytotoxicity. The modular nature of AbLecs allows for customization, where different antibody or lectin components can be swapped to address specific glyco-immune checkpoints or target various immune cell subsets, making it adaptable to a wide range of cancer types.
Experimental Evidence and Preclinical Results
In detailed experiments published in scientific journals, the team demonstrated AbLecs’ potential through in vitro and in vivo studies. They engineered AbLecs using established antibodies like trastuzumab, which targets HER2 in breast, stomach, and colorectal cancers; rituximab, directed at CD20 in lymphomas; and cetuximab, which binds EGFR in various solid tumors. These chimeras were tested on cancer cell lines, showing increased immune cell engagement and destruction of tumors, even in cells with low antigen expression.
In mouse models engineered to mimic human immune responses—with human Siglec receptors and antibody receptors—AbLecs significantly reduced lung metastases compared to antibody treatments alone. For instance, when paired with trastuzumab, AbLecs led to fewer metastatic lesions, highlighting their ability to boost the immune system’s attack on disseminated tumors. These findings indicate that AbLecs can overcome common limitations in immunotherapy, such as poor response in “cold” tumors that lack strong immune infiltration.
Potential Clinical Applications and Future Directions
The implications for cancer therapy are profound. By addressing glycan-mediated immunosuppression, AbLecs could complement existing checkpoint blockade strategies, overcoming resistance in patients unresponsive to PD-1 inhibitors. This approach holds promise for a broad spectrum of tumors, including those with high metastatic potential, and could extend to combination therapies that further amplify the immune response.
Valora Therapeutics, a biotech company spun out from this research, is advancing AbLecs toward clinical trials. While still in preclinical stages, the data suggest a path to safer, more effective treatments that minimize off-target effects by focusing on tumor-specific glycans. Potential applications span multiple cancer types, from solid tumors like breast and colorectal cancers to hematological malignancies such as lymphomas.
As researchers continue to refine this technology, challenges like optimizing dosing, ensuring long-term safety, and scaling production will need to be addressed. Future studies may explore integrating AbLecs with other modalities, such as CAR-T cell therapy or vaccines, to create synergistic effects. This could lead to more personalized treatment regimens, tailoring AbLecs to individual tumor profiles based on glycan expression patterns.
Broader Impact on Oncology and Glycobiology
This innovation underscores the power of interdisciplinary science, blending glycobiology with immunology to tackle one of medicine’s greatest challenges. Glycans, once overlooked, are now recognized as critical players in cancer biology, and targeting them opens new avenues for therapy. AbLecs not only enhance the efficacy of existing antibodies but also pave the way for novel drugs that exploit glyco-immune checkpoints.
With ongoing studies and potential trials on the horizon, AbLecs offer hope for a future where the immune system can be reliably harnessed to eradicate tumors. This could significantly reduce the burden of metastases and improve survival rates, transforming how we approach cancer and making immunotherapy a viable option for more patients. As the field evolves, such breakthroughs highlight the importance of continued investment in fundamental research to unlock innovative solutions against complex diseases.