Researchers at the University of California, San Francisco have developed a promising new cancer therapy using CRISPR activation to turn ordinary white fat cells—taken from liposuction—into energy-burning beige fat cells. These engineered cells are highly efficient at consuming glucose and fatty acids, directly competing with nearby or distant tumors for vital nutrients and effectively starving cancer growth. In mouse models, this approach significantly suppressed tumors in breast, pancreatic, prostate, and colon cancers by reducing proliferation, lowering metabolic activity, and limiting blood supply to the tumor. The method, called adipose manipulation transplantation, uses a patient’s own fat cells for personalized treatment, minimizing rejection risks, and allows reversible control. Implantation is simple, similar to fat grafting, and works even when placed away from the tumor. Published in February 2025 in Nature Biotechnology, this preclinical breakthrough offers a low-toxicity way to target cancer metabolism, with potential for future human trials and combination therapies.
Long Version
Revolutionizing Cancer Therapy: Engineered Beige Fat Cells Starve Tumors Through Metabolic Reprogramming
In the ever-evolving landscape of cancer research, a groundbreaking approach has emerged that harnesses the body’s own adipose tissue to combat tumors. Researchers at the University of California, San Francisco have pioneered a method using CRISPR activation to transform white adipose tissue—commonly obtained from liposuction—into thermogenic fat cells known as beige fat. These nutrient-hungry adipocytes outcompete cancer cells for essential resources like glucose and fatty acids, leading to tumor suppression in preclinical mouse models of breast cancer, pancreatic cancer, prostate cancer, and colon cancer. Published in February 2025 in Nature Biotechnology, this innovative therapy, dubbed adipose manipulation transplantation, represents a promising step toward personalized medicine, though human trials remain on the horizon.
Understanding Adipose Tissue: From White to Brown and Beige Fat
Adipose tissue plays a central role in metabolism, serving as the body’s primary energy storage and regulatory system. White adipose tissue, the most abundant type, stores excess calories as lipids in adipocytes, contributing to energy reserves but also linked to metabolic disorders when overgrown. In contrast, brown adipose tissue is specialized for thermogenesis, burning fatty acids and glucose to generate heat through the action of uncoupling protein 1, a mitochondrial protein that dissipates energy without producing ATP. Beige fat emerges as an intermediary: it arises from white adipose tissue under certain stimuli, exhibiting brown adipose tissue-like properties such as enhanced glucose uptake and fatty acid metabolism, making it a thermogenic fat powerhouse.
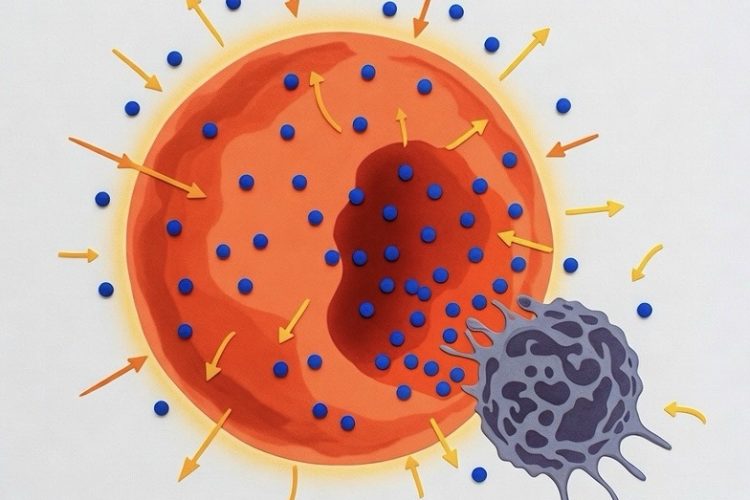
Cancer cells, notorious for their rapid proliferation, undergo metabolic reprogramming to fuel growth. They exhibit the Warburg effect—a preference for aerobic glycolysis even in oxygen-rich environments—along with heightened fatty acid metabolism. This dependency on nutrients like glucose and fatty acids creates a vulnerability that therapies can exploit. By converting white adipose tissue into beige fat, researchers amplify nutrient competition, effectively inducing cancer starvation without directly targeting tumor cells.
CRISPR Activation: The Gene Editing Tool Powering the Transformation
At the heart of this therapy is CRISPR, a revolutionary gene editing technology adapted here for activation rather than cutting. CRISPR activation employs a deactivated Cas9 enzyme fused with transcriptional activators like VP64, guided by RNA molecules to specific gene promoters. In this study, researchers targeted genes such as UCP1, PPARGC1A, and PRDM16 in liposuction-derived cells from human or mouse sources. UCP1 proved most effective, boosting oxygen consumption rates and uncoupled respiration in adipocytes, transforming them into energy-dissipating machines.
The process begins with isolating preadipocytes from white adipose tissue, differentiating them in culture, and transducing them with viral vectors carrying the CRISPR activation machinery. This results in stable upregulation of thermogenic genes, creating beige fat cells that voraciously consume nutrients. For added control, a tetracycline-inducible system allows reversible activation, ensuring the therapy can be toggled on or off as needed. This approach not only enhances the cells’ ability to burn calories but also positions them as a safe, modifiable tool for metabolic intervention.
How It Works: Nutrient Competition and Metabolic Reprogramming in Action
The engineered beige fat cells excel at metabolic reprogramming, outstripping tumors in glucose uptake and fatty acid metabolism. In vitro co-cultures using Transwell plates demonstrated that these adipocytes reduced cancer cell proliferation by 3- to 5-fold across lines from breast, colon, pancreatic, and prostate cancers. Key markers like MKI67 for proliferation and metabolic genes such as GLUT4 and GCK for glucose, or CD36 and CPT1b for fatty acids, plummeted in tumor cells, while apoptosis indicators like caspase-3 rose.
Metabolomics revealed depleted glycolytic intermediates like glucose-6-phosphate and 3-phosphoglycerate, as well as fatty acids such as oleic and palmitoleic in tumors, confirming nutrient competition as the core mechanism. High-fat or high-glucose diets negated these effects, underscoring the role of resource scarcity in cancer starvation. Additionally, for uridine-dependent pancreatic ductal adenocarcinoma, upregulating UPP1 further suppressed growth by limiting nucleotide synthesis, expanding the therapy’s adaptability to specific metabolic dependencies.
Preclinical Evidence: Mouse Models Demonstrate Tumor Suppression
In vivo validation came from xenografts in immunodeficient mice, where engineered adipose organoids—3D clusters of adipocytes—were co-implanted with cancer cells. Tumor volumes shrank by over 50%, with reduced hypoxia via CA9 marker, angiogenesis through CD31, and metabolic gene expression. Genetic models for pancreatic cancer and breast cancer mirrored these results: smaller tumors, lower insulin levels, and improved survival metrics.
Notably, implantation distant from tumors proved effective, suggesting systemic effects possibly through whole-body metabolism modulation. Patient-derived breast cancer organoids, including those from BRCA1/2 mutation carriers, showed suppressed growth when co-cultured with autologous engineered adipocytes, highlighting the therapy’s potential across diverse cancer profiles. These findings emphasize the robustness of the approach in various experimental setups.
Tailoring to Specific Cancers: Breast, Pancreatic, Prostate, and Colon
The versatility of adipose manipulation transplantation shines in its efficacy against multiple cancers. In breast cancer models, including xenografts and patient organoids, beige fat reduced proliferation and metabolic markers, even in aggressive BRCA-mutated lines. Pancreatic cancer, particularly ductal adenocarcinoma, benefited from both UCP1-driven nutrient competition and UPP1 targeting, curbing uridine reliance. Prostate and colon xenografts exhibited similar tumor suppression, with lowered glucose uptake and fatty acid metabolism, positioning the therapy as a broad-spectrum approach adaptable to different tumor types.
Implantation Strategies: From Near-Tumor to Distant Delivery
Implantation draws from established fat grafting techniques in plastic surgery. Engineered cells, formed into organoids, are mixed with Matrigel for injection near tumors or embedded in biocompatible polycaprolactone scaffolds for precise, retrievable delivery. These microwell scaffolds enhance cell retention and integration, allowing removal if needed. Both near and distant implantation suppressed tumors, with no requirement for invasive surgery, making it feasible for hard-to-access sites. This flexibility could ease clinical adoption in the future.
Personalized Medicine: Harnessing Liposuction-Derived Cells for Custom Therapy
The therapy’s strength lies in its personalized potential. Using autologous liposuction-derived cells minimizes immune rejection, enabling patient-specific engineering. For instance, fat from mastectomies was successfully converted to target breast tumors. Customizable gene activation—beyond UCP1 to nutrients like uridine—tailors the therapy to a cancer’s metabolic profile, offering a blueprint for individualized treatment in obesity-linked or nutrient-dependent cancers. This patient-centered design could integrate seamlessly with existing diagnostic tools for metabolic profiling.
Safety Profile and Challenges Ahead
Preclinical data indicate safety: no significant body weight loss, hyperglycemia, or off-target effects in mice, with lowered insulin suggesting metabolic benefits. However, challenges include ensuring long-term cell viability, preventing cancer adaptation via alternative nutrients, and assessing impacts on healthy tissues. Cachexia risks are mitigated by reversible systems, but human variability in metabolism and genetics demands careful study. Ongoing research focuses on optimizing these aspects to bridge the gap to clinical use.
Future Directions: Toward Clinical Translation
As a preclinical innovation, the therapy opens doors to combination treatments with existing drugs, multi-gene edits, or extensions to other diseases like diabetes. Researchers envision optimizing delivery, exploring endocrine effects, and initiating human trials to validate efficacy. Public and expert discussions underscore enthusiasm for this cancer-killing machine approach, though rigorous testing remains essential. With continued advancements, this method could transform how we address nutrient-dependent cancers, leveraging the body’s own cells in novel ways.
This therapy redefines cancer treatment by leveraging the body’s fat cells as allies, blending metabolism, gene editing, and implantation for a targeted, low-toxicity strategy. While early, it offers hope for a future where tumors are starved into submission.